On this page you can find details of my:
You might also be interested in:
Current Research
Inbreeding, infidelity and social environment in the superb fairy-wrens
My PhD focuses on context-dependent mate choice through an investigation of the dynamics of inbreeding & infidelity, and their potential interactions with the social environment, in a cooperatively breeding species. I use multi-generational pedigree data from a wild population of superb fairy-wrens (Malurus cyaneus), to examine the causes and magnitude of inbreeding and inbreeding depression, and to investigate explanations of infidelity, as understanding of those topics is still limited.
Past Research
Mechanisms of sperm storage in the avian female reproductive tract
My masters focused on the mechanisms of sperm storage in the avian female reproductive tract, using zebra finch (Taeniopygia guttata) and quail (Coturnix japonica) as study organisms. [Animal Behaviour MBiolSci; University of Sheffield, under the supervision of Prof Tim Birkhead and Dr Nicola Hemmings]
A bit about the project
During that year I (a) wrote a dissertation on the mechanisms of sperm acceptance, storage and release from sperm storage tubules in birds; and (b) carried out a research project developing new methods of sperm extraction and investigating the characteristics of the stored sperm.
- My dissertation focused on the mechanisms behind sperm storage within the avian female reproductive tract. During mating sperm are transferred into the vagina, but only a small proportion (<1%) of sperm reach the utero-vaginal junction (UVJ, see the diagram below), which is the storage site. Those few sperm enter the specialised storage structures, referred to as the sperm storage tubules (SSTs), where they are able to survive and stay viable for a prolonged period of time. Sperm are released from the SSTs and travel to the site of fertilisation. In birds asynchrony of copulation and ovulation is common: sperm storage helps to ensure that sperm are present at the site of fertilisation during ovulation. Over the years, many studies addressing different aspects of the storage have been published, however the mechanisms of sperm acceptance, storage and release from the SSTs are still unclear.
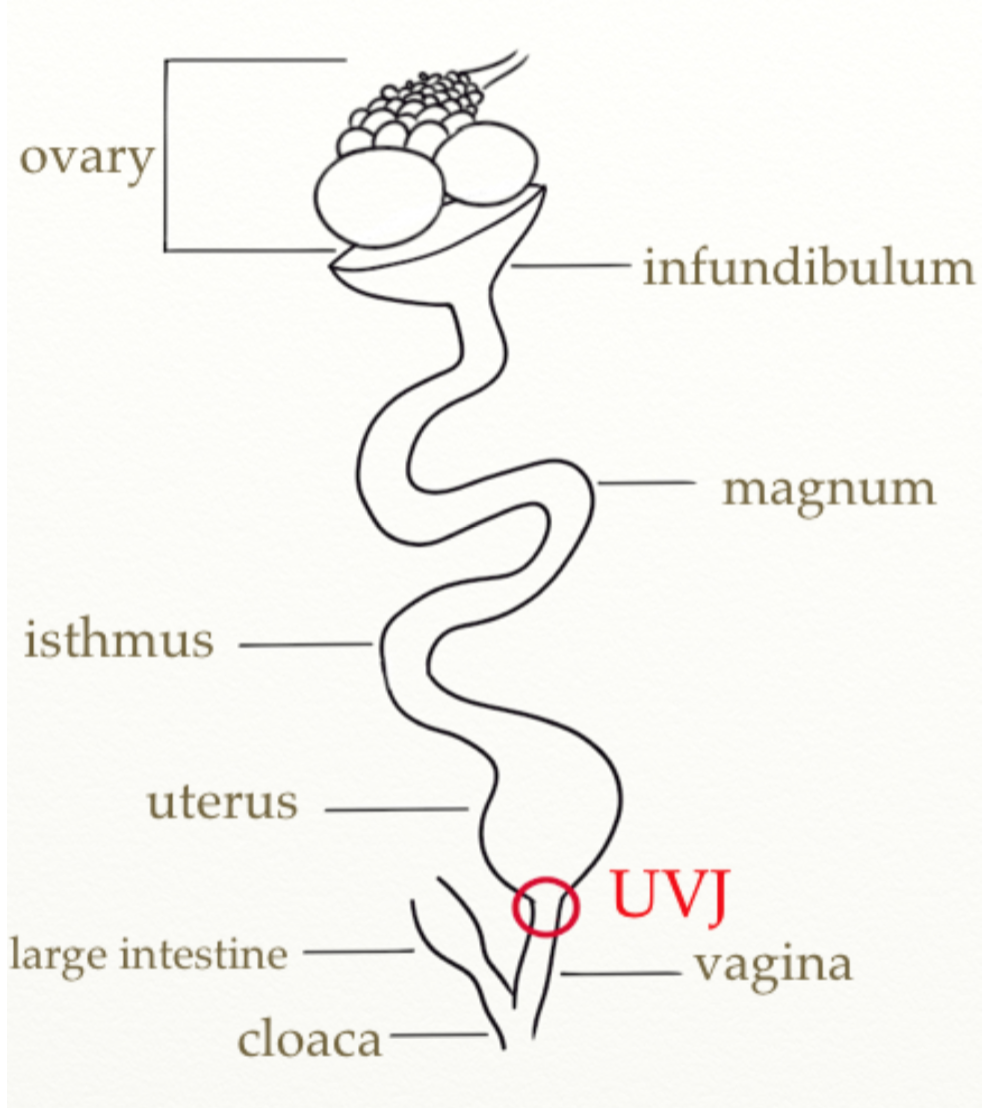
- Given that the mechanisms underlaying sperm storage are yet to be determined I set out to investigate them. Two of the biggest questions in the field of avian sperm storage are (i) whether stored sperm are deactivated during the storage period or if they are continuously swimming within the sperm storage tubules; (ii) whether a special subset of the general sperm population inseminated into the female is stored in the SSTs. It was first necessary to develop methods of sperm extraction from the sperm storage tubules without damaging the sperm, which I have achieved through developing new techniques for SST isolation and opening (after many many hours of dissecting SSTs). I have then investigated the characteristics of the stored sperm (their viability and motility) comparing the sperm extracted from the SSTs to sperm collected directly from the seminal glomera of the males.
What did I learn?
This was definitely an exercise in patience and the methods for sperm extraction took a long while to develop: while conceptually simple, they required rather high precision and great motor skills. I was even told by someone that it’s not possible to manipulate the sperm storage tubules (not to mention dissecting them) in birds smaller than blue tits - that was before I started trying and it wasn’t very encouraging! {although in all fairness, they didn’t know that I was going to be trying that} What I have learned during the previous projects has definitely helped me here - thank goodness for all the Daphnia dissections!
It was also a great lesson in adjusting my expectations and moulding into the project, going with the flow and working with what was coming out of each phase, rather than desperately trying to cling to what I’ve set out to do.
This project was my first chance to truly immerse myself in the literature of a specific field, I really felt like I knew its ins and outs. I loved that feeling, the fact that when I read a new paper I knew all the papers referenced throughout it, and I could place it well amongst the others.
Genetic basis of Seychelles warbler personalities
Independent summer research project funded by the Genetics Society, with Dr Hannah Dugdale and her (then) PhD student Hannah Edwards. I investigated the associations between SNPs in two candidate genes (DRD4 and SERT) and the bold and exploratory behaviours (behavioural data kindly provided by Hannah E.) in the Seychelles warbler (Acrocephalus sechellensis).
This project has resulted in a publication which I’m first co-author on, you can find it here.
A bit about the project
Personalities are between-individual differences in behaviour that are consistent through context and time. Investigating the genetic basis of personalities might provide us with insight into maintenance of this variation. Both the dopamine receptor D4 (DRD4), and serotonin transporter (SERT) have been previously identified as promising candidate genes for personality in birds and therefore they were an obvious starting point: previous studies of wild bird populations have shown that exploratory and bold behaviours are associated with polymorphisms in both of those genes. We found that DRD4 was completely monomorphic, while SERT had four polymorphisms (five haplotypes). However, neither the polymorphisms, nor the haplotypes were associated with either bold or exploratory behaviours in our population (see the paper for details).
What did I learn?
This was the first time I have worked in a molecular ecology lab and so from the technical side of things it was all new to me. I did everything from designing and testing primers, through gel electrophoresis and PCRs all the way to gene sequencing. I discovered that I’m rather good at lab work, except for working with the centrifuge which clearly hated me - I could balance the plates to perfection and yet it still wouldn’t run. Then one of the other people in the lab would pop along and stick a bit of masking tape on one of the plates and it would run just fine. The workings of this centrifuge remain a mystery to me, but I had pipetting covered!
Before this project I never considered how much waiting around there is while things (PCRs, sequencing etc.) run: time management is definitely crucial here.
I have also learned to use several programs, including Codon Code Aligner and fastPHASE to reconstruct haplotypes (which required DOS, who knew that DOS was used for anything those days!). This project is also when I taught myself the basics of R in order to carry out some data analysis - this is also were I had to teach myself about mixed effects models. Learning both the tools and the concepts at the same time was tough, and I’d strongly suggest playing with the programs early on, before you really need them.
Phenotypic plasticity and chitin allocation in Daphnia
Independent summer research funded by the British Ecological Society, with Dr Andrew Beckerman. I developed protocols for Daphnia head and gut isolation, embedding, sectioning and staining.
A bit about the project
In arthropods, the carapace and moulting process involve the synthesis and degradation of chitin. Chitin genes are also likely involved in the defence against predators, pathogens and parasites. We wanted to measure chitin allocation patterns in Daphnia facing predator and immune stress. Daphnia can change their morphology and life history in response to predators and we also know that immune function in arthropods is tightly linked to chitin (e.g. petritrophic gut membrane). We therefore wondered whether there would be a trade-off in chitin allocation in Daphnia under immune and predation stress.
We predicted that daphnids exposed to pathogens will have less chitin allocated to carapace and more to guts, while daphnids exposed to predators will have more chitin allocated to carapace and less to guts.
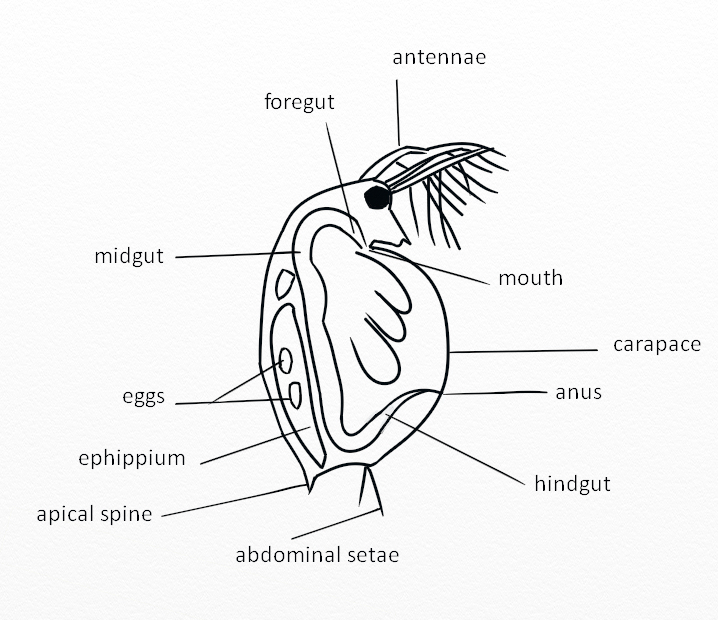
What did I learn?
I have volunteered in Dr Beckerman’s lab the previous summer, so I was familiar with working in a wet lab and managing a Daphnia population. This project required a lot of dissections - the first 200 or so daphnids were really quite tough, as I haven’t used a microscope to extensively manipulate small specimens before that. I’ve managed to go from squishing them to dissecting and manipulating their parts with ease, but I have to admit that it was a very frustrating process at times. It’s annoying when your body doesn’t develop the motor skills as quickly as you’d like it to - perseverance is key. Those skills and experience were invaluable in my later projects, especially the work on sperm storage tubules.
Once I had the dissections down I went onto the rest of the project and learned to use a bunch of equipment which we, us undegrads, weren’t normally allowed to touch. Getting exposure to lab practices and protocols is great, even if you are just getting trained up to use more sensitive weighing scales or water baths. I ended up being the only person who could program the drying oven in our lab :D I also learned digestions, stained specimens with various dyes and was even trained up on a microtome so that I could section Daphnia samples after embedding (following Technovit protocols).
Questions? Get in touch!
I love discussing my research, if you have any questions or observations, don’t hesitate to email me. You can also connect with me on Twitter.